1. Introduction to non-alcoholic steatohepatitis
Non-alcoholic steatohepatitis (nonalcoholic steatohepatitis, NASH) is a common liver injury, a metabolic disorder, accompanied with varying degrees of inflammation and fibrosis that will eventually evolve into end-stage liver disease (e. g., cirrhosis, liver cancer), with histopathological abnormalities similar to alcoholic steatohepatitis. Histopathological features included steatosis, evidence of hepatocellular injury, mixed inflammatory lobular infiltrates, and variable fibrosis. Secondary theory is more widely accepted a explanation of the pathogenesis of NASH hypothesis, a blow is caused by fat accumulation in the liver, steatotic liver cells are more vulnerable to the second damage, lead to a series of damage of liver cells including inflammation, apoptosis, fibrosis and cancer, start the second blow of the main factors including oxidative stress, lipid peroxidation, promote inflammatory factors, mitochondrial dysfunction, liver microcirculation dysfunction, etc.
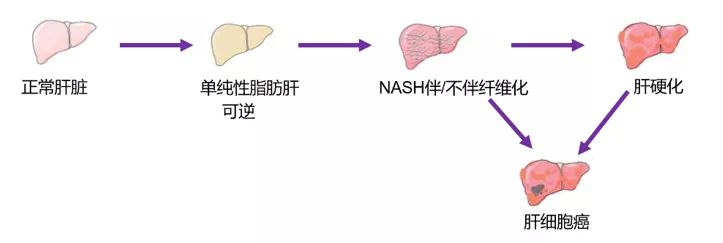
Figure 1 Formation process of nonalcoholic steatohepatitis
2. Modeling methods for common models
According to the pathogenesis of fatty liver, the construction principle can be divided into the following five categories:
1. By changing the nutritional structure of high-fat feed, making the animals ingest or synthesize excessive fat, or making the special treatment of high-fat feed, the animal liver is damaged, and a fatty liver model is constructed;
2. Looking for naturally occurring specific gene mutations, or targeted gene modification to induce obesity, liver steatosis and other symptoms;
3. Use drugs or hormones to intervene in the process of animal fat synthesis and metabolism, and imitate the symptoms of fatty liver;
4. The induction of high-fat feed with gene knockout or a mixture of drug models shortens the construction cycle of fatty liver model and makes the symptoms more comprehensive;
5 Use natural animal cells as a model of fat disorder without additional drug agents.
According to the model making method, animal models of non-alcoholic fatty liver disease can be divided into diet induction, chemical induction, gene editing, etc.
1 Diet induction
1.1 Nutrition-dysregulated fatty liver model
1.1.1 High-fat diet (Highfatdiet, HFD) model
(1) HFD is the most basic model of non-alcoholic fatty liver disease, and this model roughly simulates the western diet. HFD contains more fat synthesis raw materials, which can promote liver lipogenesis and excessive accumulation of fat, thus leading to fatty liver.
Because C57BL / 6 mice are sensitive to high-fat diet and are prone to form hyperlipidemia, they are commonly used to replicate NASH models.
After long-term feeding of HFD, the animals will mainly appear obesity, IR, simple steatosis and other symptoms. The common HFD contains 71% fat, 11% carbohydrate, and 18% protein.
The severity of inflammation in HFD is proportional to the treatment time. Currently, the induction duration of most studies of non-alcoholic steatohepatitis (NASH) models is 12 to 20 weeks.
Mouse models fed a high-fat diet mimic the histopathology and pathogenesis of human NAFLD, as it has the main features observed in patients with NAFLD, including obesity and insulin resistance. Therefore, this model is suitable for evaluation to evaluate weight-loss, low-fat diet and therapeutic agents to improve insulin resistance. From the pathogenesis, the development of the course of disease and eventually histomorphological changes and human tissue differences, and animal mortality, cost, the most commonly used model for diet induced type, high-fat diet model also has certain limitations, such as molding time is long, at least two months, and with the different animal germline and diet formula present diversity.
(2) recent studies have also shown that high fat feed (HFD, formula for ordinary feed 53.5%, fructose 20%, lard 20%, 5% cholesterol, 1% salt, sodium cholate 0.5%) combined with different concentrations of glucan sulfate (DSS) water mice non-alcoholic steatohepatitis (NASH) animal model, giving 2% DSS can accelerate the establishment of high fat diet induced mice NASH model, can be the molding time reduced to 2 weeks, greatly shorten the molding time.
1.1.2 Atherosatogenic diet (ATH) model
ATH contains 0.5% cholate and 1.25% cholesterol and is commonly applied to mice to build an atherosclerotic plaque model.
ATH was found to cause dyslipidemia in C57BL / 6 mice. At the same time, within 6-24w, ATH also triggered NASH, and the longer the molding time, the more serious the pathological state, such as the occurrence of hepatocyte massive bubble fatty change, inflammation and liver fibrosis. Furthermore, the mice also exhibited elevated levels of alanine aminotransferase, total cholesterol, and triglycerides after 6w.
However, ATH does not cause weight gain, and there is no obvious insulin resistance, therefore, it is different from human NASH and becomes a defect in this model.
1.1.3 High-fat + atherogenic diet (HFD + ATH) model
HFD + ATH was based on a high-fat diet, supplemented with 0.5% cholate and 1.25% cholesterol. This model combines the advantages of the high-fat model and the ATH model to effectively promote the further development of nonalcoholic steatohepatitis, with the main pathological features being insulin resistance, oxidative stress, and hepatic stellate cell activation.
HFD + ATH (60% fat + 1.25% cholesterol + 0.5% cholate) was compared with the choline-methionine-deficient diet. Both had similar NASH pathological characteristics. HFH + ATH-induced C57BL / 6 mice showed increased hepatic cholesterol and free fatty acids, whereas the choline-methionine-deficient diet had increased triglyceride levels in the liver.
1.1.4 High-fat + cholesterol diet model
A high fat + cholesterol diet is a high-fat diet with 21% butter and 0.2% cholesterol.
After short-term feeding of a high-fat + cholesterol diet, C57BL / 6J mice developed only hepatic steatosis. However, female ldlr- / -and APOE2ki mice would exhibit severe inflammation and steatosis characterized by macrophage infiltration and enhanced NF-kappaB-mediated signals. Male ldlr- / -and APOE2ki mice also developed severe liver inflammation, but no steatosis.
In addition, a Western diet rich in n-6-PUFA soybean oil (100g soybean oil contains 25g of n-6-polyunsaturated fatty acids) and 0.75% cholesterol. After C57BL / 6 mice were given this diet for 20w, mice exhibited balloon hepatic steatosis, oxidative stress, inflammatory cell infiltration, Kupffer activation, and liver fibrosis with NAFLD features of body weight gain and insulin resistance.
1.1.5 High-fat + high-sugar diet (ALIOS) model
ALIOS is a mouse model induced by high-fat and high-glucose diet. ALIOS was fed on high-fat diet (containing 30% trans fatty acids) and fructose glucose syrup. After 16w, C57BL / 6 mice had significant steatosis, with necrotizing inflammation and increased alanine transaminase levels.
Compared with high-fat diet only (containing 30% trans fatty acids), the ALIOS group significantly increased body weight, increased food intake and increased insulin resistance, but little changes in steatosis and alanine aminase levels.
It has been reported that by using western diet (Tekladdiets, TD.120528), with low dose of CCl4 intraperitoneal injection, C57BL / 6 mice can rapidly evolve from simple fatty liver to steatohepatitis liver, liver fiber and hepatocellular carcinoma, almost in line with the pathological process of human NAFLD.
1.1.6 High fat + high fructose + high cholesterol diet (AMLN, GAN) model
To compensate for the previously reported ALIOS mouse model, the pathology of the liver was assessed more objectively by a high fat + high fructose + high cholesterol (AMLN) dietary model, containing 40% fat (about 18% trans fatty acids), 22% fructose and 2% cholesterol.
Both C57BL / 6 and ob / ob mice, simultaneously fed with AMLN (26 – 30w), showed significant hepatic steatosis, hepatic lobular inflammatory cell infiltration, and swelling of hepatocyte morphology.
It is noted that C57BL / 6 mice were fed 26 – 30w before having the above pathological features appeared. Compared with C57BL / 6 mice, ob / ob mice showed significantly higher levels of triglyceride and alanine aminotransferase after feeding AMLN12w.
Another high fat + high cholesterol + high fructose diet with 41% fat, 30% fructose and 2% cholesterol can very well mimics the habit of high fat, high glucose and high cholesterol in the Western diet. The diet model is more widely applicable and has been used to establish non-alcoholic steatohepatitis models in mice with multiple genotypes.
The study reported that the study proposed a GAN diet model containing 40% fat (no trans fatty acids), 22% fructose, 10% sucrose and 2% cholesterol. After feeding GAN 8-16w, ob / ob mice showed significant weight gain and were very similar to the pathological features of human NASH. Furthermore, there was also agreement between the pathological characteristics of ob / ob mice on the AMLN diet, but weight gain was more pronounced in ob / ob mice on the GAN diet.
In contrast, C57BL / 6 mice required a long feeding period (usually after 28w) to induce a NASH model consistent with the above.
1.1.7 Choline-methionine deficiency diet (MCD) model
The Choline-methionine-deficient diet contained 40% sucrose and 10% fat, but contained no methionine and choline. Initially within 2-4w mice develop major features of the NAFLD pathological phenotype, such as macrovesicular fatty changes in mouse hepatocytes, and subsequently significant fibrosis.
Although the MCD model is an important tool to study NASH and used for early drug screening, the MCD model also has certain limitations, such as choline-methionine diet preparation tedious, does not conform to the human diet rules, is also completely different from the actual metabolic status of human NASH patients, lack of systemic insulin resistance characteristics of human NASH.
Related studies have found that the MCD model presents low serum insulin, fasting glucose, leptin and triglyceride levels. Unlike NASH patients, who were mostly obese, the MCD model showed severe weight loss and a cachectic state compared with normal controls.
1.1.8 Choline-deficient L-amino acid diet (CDAA) model
The CDAA diet contained 68.5% carbohydrate, 17.4% protein, and 14% fat. The CDAA diet induction model is similar to the MCD mouse model, which suppresses fatty acid oxidation in hepatocytes, increasing lipid synthesis, oxidative stress and inflammation, ultimately leading to fibrosis.
Chronic mice fed CDAA also showed significant increases in weight gain, insulin resistance and lipid levels. In addition, CDAA fed mice have experienced metabolic symptoms for a long time, which is currently limited to liver disease studies, and should not be used separately in NASH related studies.
2, Drug-induced fatty liver model
2.1 Streptozotocin (STZ) induction model
Streptozotocin induction model is commonly seen in the experimental model inducing type 2 diabetes, C57BL / 6 mice given 200 μg/L in combination with high-fat diet feeding, steatohepatitis, fibrosis and even liver cancer within approximately 20w, can be used as a mouse model of NAFLD.
STZ induction model mice showed simple hepatic steatosis at 6 weeks of age, lobular inflammation and extensive hepatocytes at 8w, hepatic fat deposition to fibrosis at 8 – 12w, and high incidence of hepatocellular carcinoma in mice at 20w. Transaminase and blood glucose concentrations were consistently increased in mice during mold production.
This model can induce oxidative stress, inflammation, fibrosis, and hepatocyte apoptosis, consistent with the histopathology of NAFLD. But unlike insulin resistance in human NAFLD, streptozotocin disrupts islet β -cell function without causing systemic insulin resistance.
Therefore, this also limits the use of this model in preclinical drug studies.
2.2 Carbon tetracloride (CCl4) induction model
The CCl4 alone induction model only showed liver fibrosis, without developing lesions such as obesity and insulin resistance, and therefore does not belong to the NAFLD model.
Therefore, CCl4 usually needs to prepare NASH models combined with diet induction models, such as CCl4 can promote the development of high-fat diet on non-alcoholic steatohepatitis and liver fibrosis.
3 Model of the induction of gene editing
3.1 ob / ob and db / db
Leptin expressed by obesity gene (OB) regulates body weight and fat deposition by affecting metabolism and appetite. Its content is inversely proportional to the degree of fat deposition. Leptin receptor is a protein encoded by diabetes-related gene (db gene), and the loss of leptin receptor encoded by DB gene is easy to cause hyperglycemia or IR.
The ob / ob or db / db mice first have recessive mutations, congenital lack of dominant OB or DB gene, unable to encode leptin or leptin receptor, it is characterized by increased lipid accumulation in the liver, not spontaneous inflammation and fibrosis, showing obvious metabolic characteristics of fatty liver, is a good genetic model of fatty liver, the difference is that ob / ob mice can be resistant to liver fibrosis, while db / db mice are not resistant to liver fibrosis.
However, leptin resistance caused by congenital leptin deficiency or genetic mutations is not prevalent in obese or NASH patients, and thus the ob / ob and db / db mouse models have limited ability to reflect the causes of obesity or NASH in animals.
3.2Dicer1 gene knockout
In order to develop an animal disease model similar to that of human non-alcoholic steatohepatitis (Non-alcoholic steatohepatitis, NASH), a novel NASH mouse model was constructed using liver-specific Dicer1 knockout (KO) mice induced on a high-fat and high-glucose diet for 12 weeks.
3.3 129S1 / SvImJ and C57BL / 6J hybrid mice
Syngeneic strain-induced nonalcoholic fatty liver models using two common mouse strains, 129S1 / SvImJ and C57BL / 6J crosses, with a simple high-fat diet consuming high fructose and glucose (Western diet sugar water (WDSW)), diet-induced mouse models of nonalcoholic steatohepatitis (NASH) and liver cancer have been developed between two mouse strains (129S1 / SvImJ and C57Bl / 6J). This model mimics all the physiological, metabolic, histological, transcriptomic gene features and clinical endpoints of human NASH, and can facilitate preclinical development of therapeutic targets for NASH.
4 Composite model
Gene editing models are unable to develop fatty liver independently, so many animal models combine naturally occurring gene mutations or targeted gene modification and diet induction to make the histopathology and pathophysiology of the model closer to that of animal fatty liver.
for instance:
(1) Female Alms11 mutant (foz / foz) mice fed an atherogenic diet (23% fat, 45% carbohydrate, 20% protein, 0.2% cholesterol) for 16 weeks, foz / foz mice weighed> 60g, characterized as hyperinsulinemia, diabetes, hypertension, hypercholesterolemia, low adiponectinemia and NASH with fibrosis, and this mixed model better mimics human fatty liver status [7].
(2) On MCD diet (10% fat, 40% sucrose, and 10, and 4 weeks, severe fatty infiltration in the liver, increases in thiobarbituric acid reactant levels and nitrite, and db / db mice had significantly higher serum ALT levels and more severe hepatic inflammation and fibrosis than ob / ob and wild-type mice. As steatosis in liver tissue and oxidative stress are closely related, these changes may contribute to NASH in animals.
Studies have also fed ddb / db mice, which has observed that iron overload in the diet can accelerate the development of animal fatty liver to NASH.
(3) A study on 30-week-old OLETF rats fed a HF diet containing 60% fructose has found that they produced symptoms such as dyslipidemia and inflammation, and the expression of BiP, p-IRE 1 α and t-IRE 1 α protein was increased significantly, indicating that the ER stress pathway was activated.
According to the purpose of the experimental research, the investigators should select the animal model that meets the characteristics of human fatty liver formation process and lesion, short mold formation time, low animal mortality rate, good reproducibility, and simple operation method.
3. Currently marketed drugs
Currently, with almost no treatment for NASH and patients having huge unmet clinical needs, there is an urgent need to develop effective therapies to address this global health problem. NASH is a hot field for new drug research and development, and new drugs have emerged in recent years. Current drugs used for NASH treatment include nuclear receptor agonists such as FXR agonists, PPAR agonists, chemokine receptor inhibitors, thyroid hormone receptor- β agonists, and GLP-1, FGF 21, or SGLT 2 inhibitors.
PPAR receptor is a nuclear receptor that regulates metabolic homeostasis, cell differentiation and immune inflammation, and is a popular therapeutic target for NASH in recent years. A PPAR α / γ dual agonist, saroglitazar, has been first approved in India. In March 2020, the Indian Medical Products Agency approved Saroglitazar for the treatment of NASH, which is also the first drug approved for the treatment of non-cirrhotic NASH, but the indication has not yet passed the US Food and Drug Administration (FDA) and the European Union Medicines Agency, which has not been widely accepted internationally.
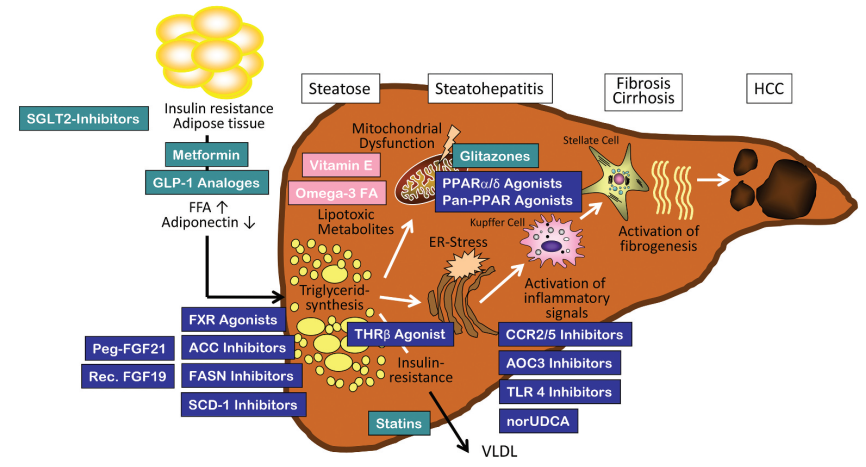
4. Action target
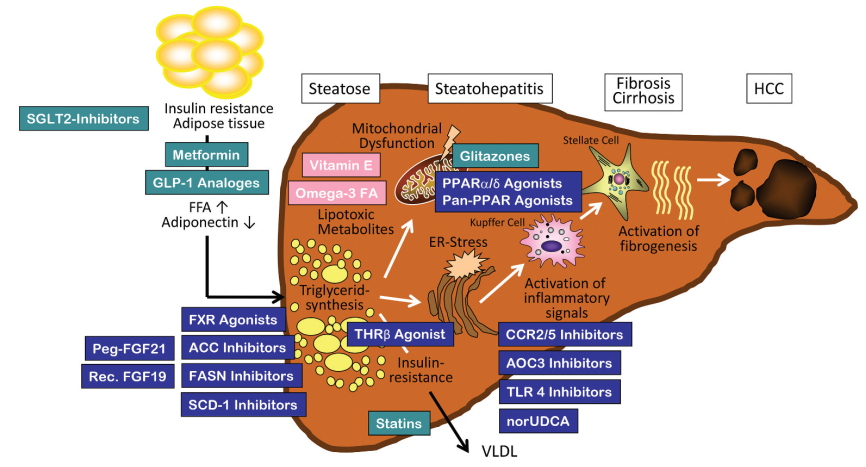
Figure 2. Pathogenesis and new therapeutic targets of NASH
1. The PPAR receptor
PPAR receptor is a nuclear receptor that regulates metabolic homeostasis, cell differentiation and immune inflammation, and is a popular therapeutic target for NASH in recent years.
2 and that of the thyroid hormone β receptor
The Thyroid hormone β receptor is currently a relatively promising therapeutic target. Thyroid hormone receptor β agonists improve NASH mainly by promoting liver lipid metabolism and reducing lipid toxicity. MGL-3196 and VK2809 have entered the late clinical stage and show a significant effect of reducing liver fat content.
3. SCD-1
Stearoyl-Coenzyme A desaturase 1 (SCD-1) is a key enzyme playing a role in hepatic lipogenesis, converting saturated fatty acids to monounsaturated fatty acids. Recently, SCD-1 has become a major research target for NAFLD dietary metabolic models.
4. XBP1
XBP 1 is a transcription factor that is upregulated in the liver tissue of patients with non-alcoholic steatohepatitis (NASH). Conditional knockout of Xbp 1 in hepatocytes resulted in reduced lipid accumulation in mice, and the genetic deletion of Xbp 1 in macrophages ameliorated vegetative steatohepatitis and fibrosis in mice. Pharmacological inhibition of XBP 1 may prevent steatohepatitis and fibrosis.
5. The ALOX12ACC1-axis of the
On the basis of multi-omics studies and cross-species multilayer validation, researchers have shown that arachidonic acid 12-lipoxygenase (ALOX12) -acetyl Coenzyme A (CoA) carboxylase 1 (ACC1) axis is the core driver of NASH progression. Previous studies have shown that ALOX12 is involved in liver injury, such as liver ischemia and reperfusion injury and Ccl 4, ALOX12 enzyme inhibitor ML355 can effectively improve thrombosis, platelet aggregation and islet dysfunction. Although ALOX12 is involved in the above studies mainly through its enzymatic activity producing 12-hydroxyeicosapentraenoic acid (12-HETE), our companion studies showed that ALOX12 promotes NASH progression in an enzymatic activity-independent manner. Previous reports have identified ACC as an attractive therapeutic target for NASH because of the robust improvement of hepatic steatosis and fibrosis by ACC inhibitors in both experimental observations and clinical trials.
5. reference documentation
[1] Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al.Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease.Gastroenterology 2015;149: 389–397e310.
[2]Paradies G, Paradies V,Ruggiero FM,et al.Oxidative stress,cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease[J].World J Gastroenterol, 2014,20(39):14205-14218.
[3]Santhekadur PK, Kumar DP, Sanyal AJ.Preclinical models of non-alcoholic fatty liver disease.J Hepatol.2018 Feb;68(2):230-237. doi: 10.1016/j.jhep.2017.10.031. Epub 2017 Nov 9. PMID: 29128391; PMCID: PMC5775040.
[4] Wan Xiaoyu, Zeng Junhao, Zhang Wanyi, Han Yu, Yang Tianxin, Wang Yaqin, Zhou Yongqin, Liu Chaoqi. Establishment of a mouse model of nonalcoholic steatohepatitis [J]. Chinese Journal of Immunology, 2020,36 (16): 1925-1930.
[5] Su Xiaoou, Chen Jing, Qi Xinming, Zhou Xiangmei, Qiao Junwen, Ren Jin. Establishment of a novel mouse model of nonalcoholic steatohepatitis [J]. Chinese Veterinary Journal, 2019,55 (08): 87-91 + 95 + 132.
[6]Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D Jr, Warncke UO, Wijesinghe DS, Sanyal AJ.A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer.J Hepatol.2016 Sep;65(3):579-88.
[7] Larter CZ, Yeh MM, Van Rooyen DM, Teoh NC, Brooling J, Hou JY, et al.Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice.J Gastroenterol Hepatol 2009;24: 1658–1668.
[8] Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, et al.Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis.Gastroenterology 2011;141: 1393–1403.
[8]RATZIU V, HARRISON SA, FRANCQUE S, et al.Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and-δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening[J].Gastroenterology, 2016, 150(5): 1147-1159. e5.
[9] SHARMA M, PREMKUMAR M, KULKARNI AV, et al.Drugs for non-alcoholic steatohepatitis (NASH): Quest for the holy grail[J].J Clin Transl Hepatol, 2021, 9(1): 40-50.
[10]Monika Rau, Andreas Geier.An update on drug development for the treatment of nonalcoholic fatty liver disease – from ongoing clinical trials to future therapy [J].Expert Review of Clinical Pharmacology.2021, 14(3): 333-340.
[11] RATZIU V, HARRISON SA, FRANCQUE S, et al.Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and-δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening[J].Gastroenterology, 2016, 150(5): 1147-1159. e5.
[12] ALKHOURI N.Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: Rationale for the development of resmetirom (MGL-3196)[J].Expert Opin Investig Drugs, 2020, 29(2): 99-101.
[13] SAFADI R, KONIKOFF FM, MAHAMID M, et al.The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease[J].Clin Gastroenterol Hepatol, 2014, 12(12): 2085-2091. e1.
[14]Wang Q, Zhou H, Bu Q, Wei S, Li L, Zhou J, Zhou S, Su W, Liu M, Liu Z, Wang M, Lu L.Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis.J Hepatol.2022 Aug;77(2):312-325.
[15]X.J.Zhang, Z.G.She, J.Wang, D.Sun, L.J.Shen, H.Xiang, X.Cheng, Y.X.Ji, Y.P.Huang, P.L.Li, X.Yang, Y.Cheng, J.P.Ma, H.P.Wang, Y.Hu, F.Hu, S.Tian, H.Tian, P.Zhang, G.-N.Zhao, L.Wang, M.L.Hu, Q.Yang, L.H.Zhu, J.Cai, J.Yang, X.Zhang, X.Ma, Q.Xu, R.M.Touyz, P.P.Liu, R.Loomba, Y.Wang, H.Li, Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis.Sci.Transl.Med.13, eabg8117 (2021).
[16]C.Dai, H.Li, Y.Wang, S.Tang, T.Velkov, J.Shen, Inhibition of oxidative stress and ALOX12 and NF-κB pathways contribute to the protective effect of baicalein on carbon tetrachloride-induced acute liver injury.Antioxidants 10, 976 (2021).
[17]F.Yang, Y.Zhang, H.Ren, J.Wang, L.Shang, Y.Liu, W.Zhu, X.Shi, Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis.J.Exp.Clin.Cancer Res.38, 489 (2019).
[18]X.J.Zhang, Z.G.She, J.Wang, D.Sun, L.J.Shen, H.Xiang, X.Cheng, Y.X.Ji, Y.P.Huang, P.L.Li, X.Yang, Y.Cheng, J.P.Ma, H.P.Wang, Y.Hu, F.Hu, S.Tian, H.Tian, P.Zhang, G.-N.Zhao, L.Wang, M.L.Hu, Q.Yang, L.H.Zhu, J.Cai, J.Yang, X.Zhang, X.Ma, Q.Xu, R.M.Touyz, P.P.Liu, R.Loomba, Y.Wang, H.Li, Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis.Sci.Transl.Med.13, eabg8117 (2021)
[19]C.Dai, H.Li, Y.Wang, S.Tang, T.Velkov, J.Shen, Inhibition of oxidative stress and ALOX12 and NF-κB pathways contribute to the protective effect of baicalein on carbon tetrachloride-induced acute liver injury.Antioxidants 10, 976 (2021).
[20]H.He, R.Adili, L.Liu, K.Hong, M.Holinstat, A.Schwendeman, Synthetic high-density lipoproteins loaded with an antiplatelet drug for efficient inhibition of thrombosis in mice.Sci.Adv.6, eabd0130 (2020).